
We hope you had a lovely Christmas and New Year.
We are excited to bring to you a recent human study we conducted which qualified Vitamin D3V® as a bioavailable source of Vitamin D3. The study was completed in Ireland through Research & Development business AnaBio Technologies Ltd together with University College Dublin.
Study Highlights
- The study was performed on 10 healthy volunteers using a daily dose of 600iu Vitamin D3V®.
- All study participants saw a significant increase in plasma Vitamin D levels over the baseline.
- The average plasma Vitamin D increases were from 43.43 to 77 nmol/L (33nmol/L). This marks an average increase of approximately 77.3% above baseline for participants.
- The results were statistically significant, with a p-value using a paired t-test of 0.002.
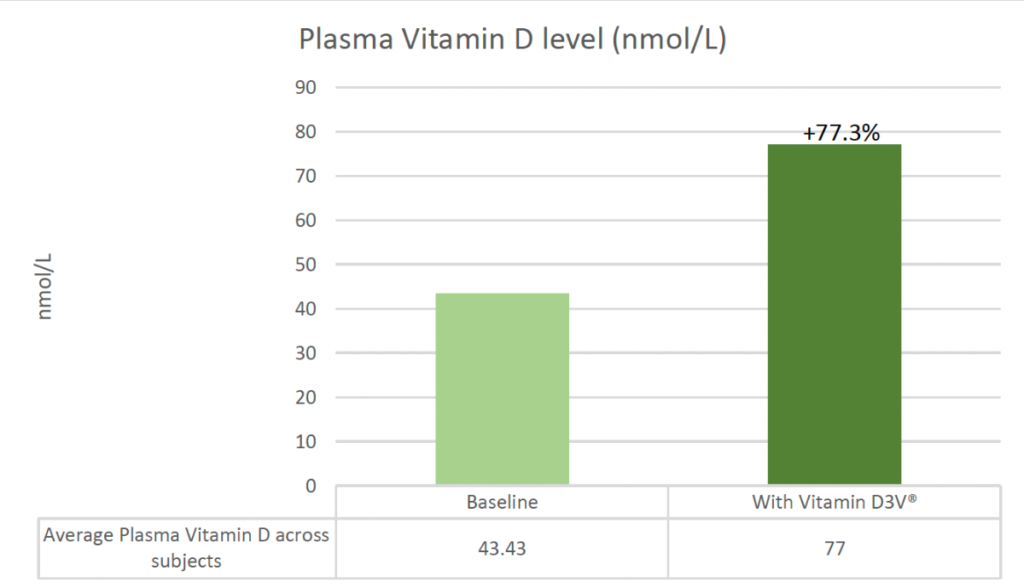
The study confirmed that Vitamin D3V® is a bioavailable source of Vitamin D3, supporting the existing analytical data.
We also would like to bring to your attention that the import of Lanolin-origin Vitamin D3 into Europe could be banned on 11th June 2021. The European Commission updated the Q&A section of its Import of Composite Products into the EU document, with a comment specifically regarding Lanolin-origin Vitamin D3.
To find out more, click the button below.
If you have any questions regarding anything you have seen, please feel free to get in contact.
